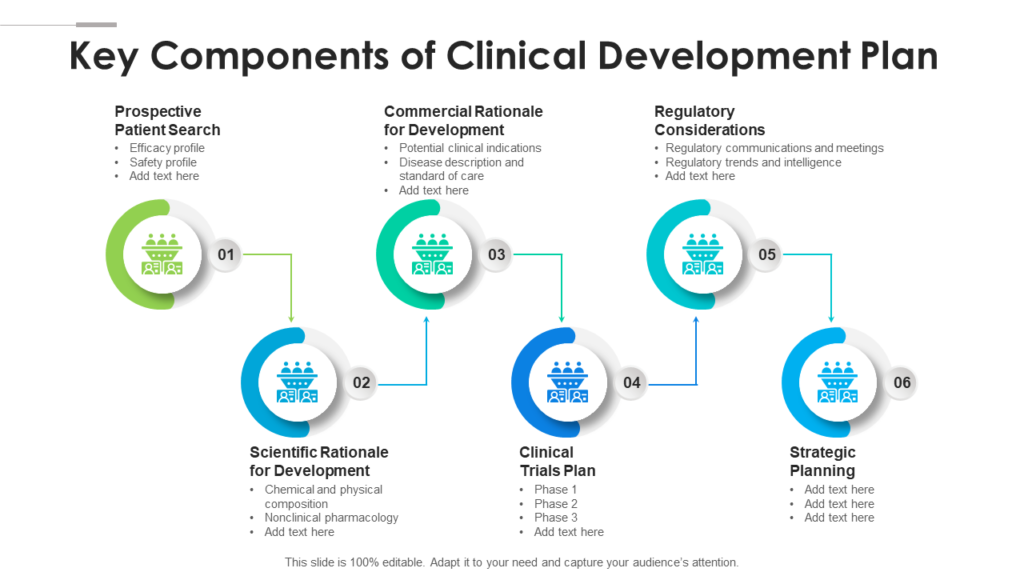
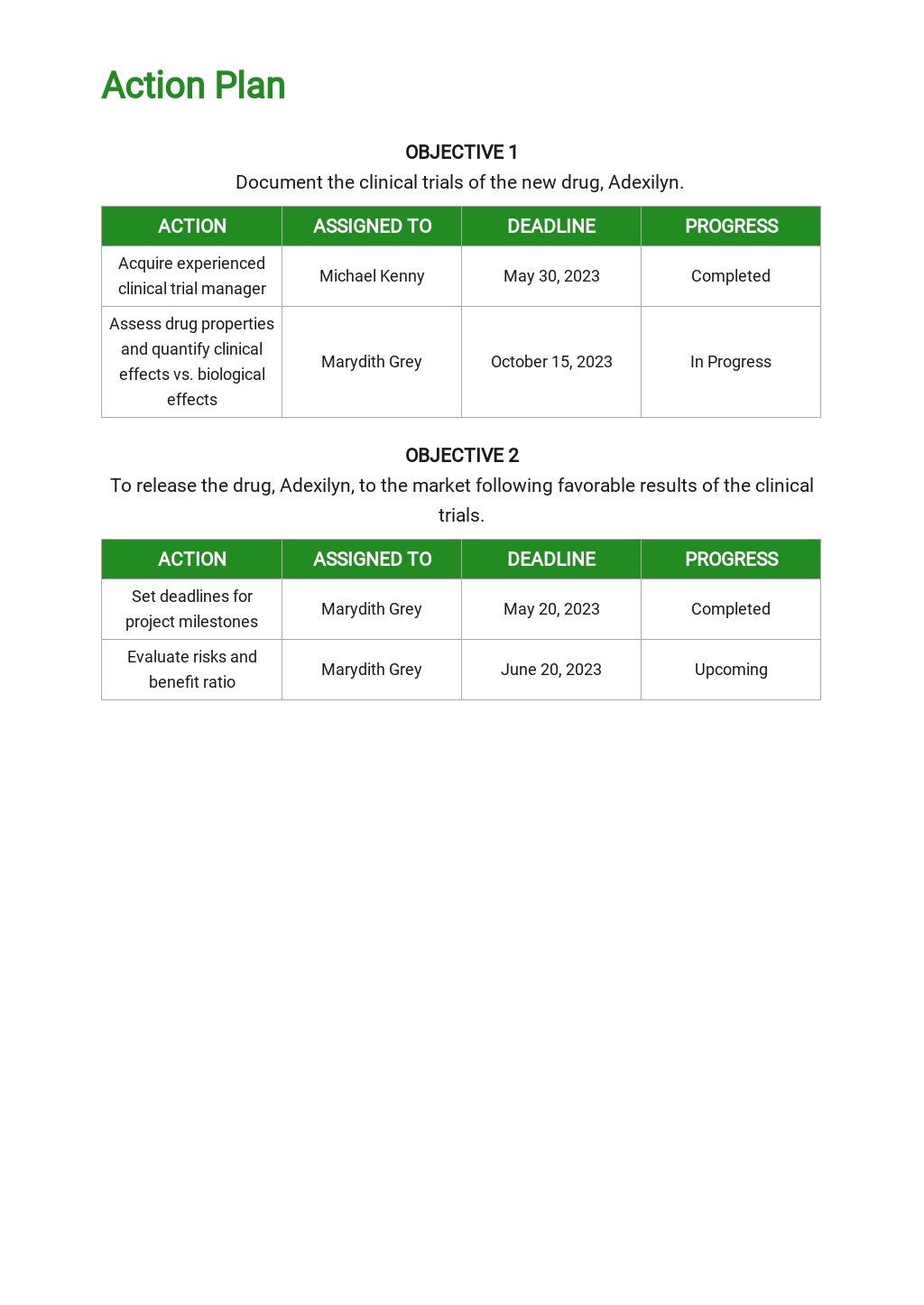
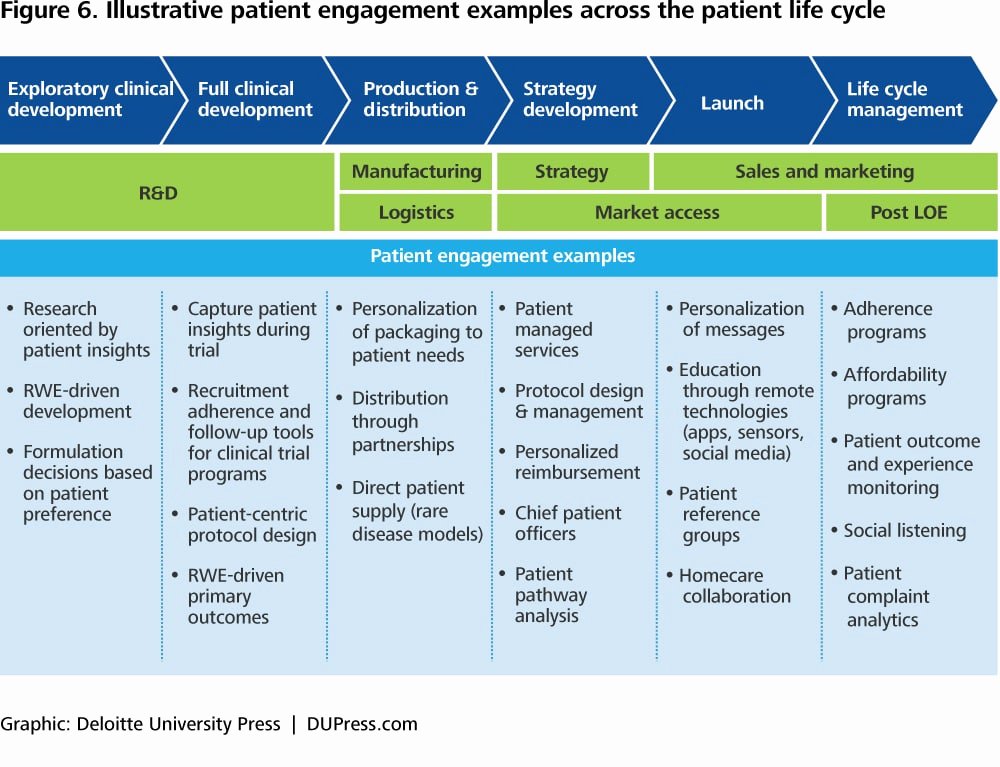
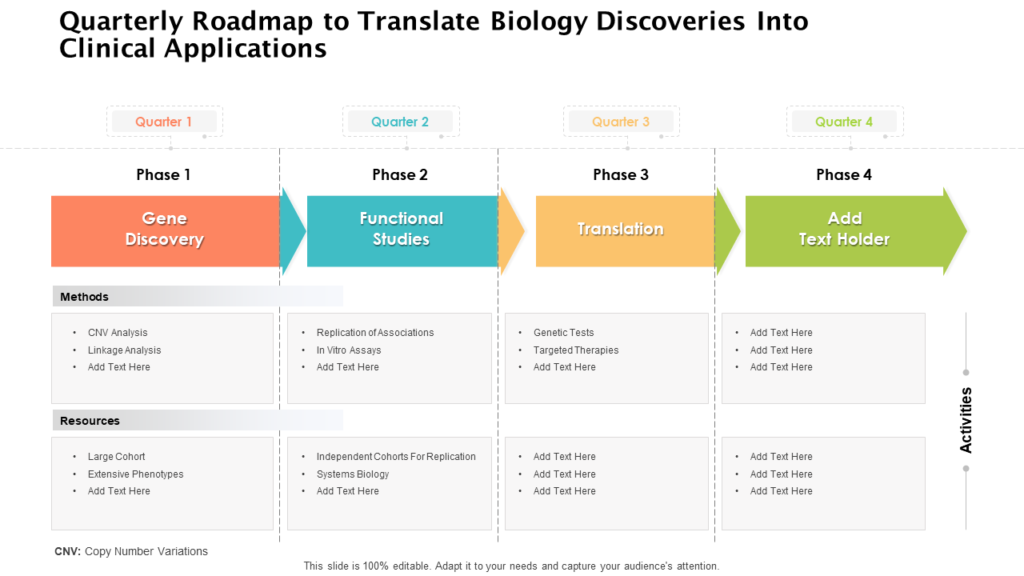
Clinical Development Plan Template - The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation. Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020.
Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020. The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation.
Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020. The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation.
Top 20 PowerPoint Templates to Create a Clinical Development Plan
The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation. Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020.
Clinical Development Plan Template
The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation. Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020.
Clinical Development Plan Template Fda
Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020. The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation.
Clinical Development Plan Template in Google Docs, Pages, Word
The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation. Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020.
Clinical Development Plan Template Peterainsworth
Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020. The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation.
Clinical Development Plan Template
The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation. Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020.
30 Clinical Development Plan Template Hamiltonplastering
Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020. The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation.
Top 20 PowerPoint Templates to Create a Clinical Development Plan
The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation. Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020.
Clinical Development Plan Template Google Docs, Word, Apple Pages
Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020. The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation.
Learn How To Plan And Document Your Medical Device's Clinical Evaluation According To The Mdr And Iso 14155:2020.
The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation.