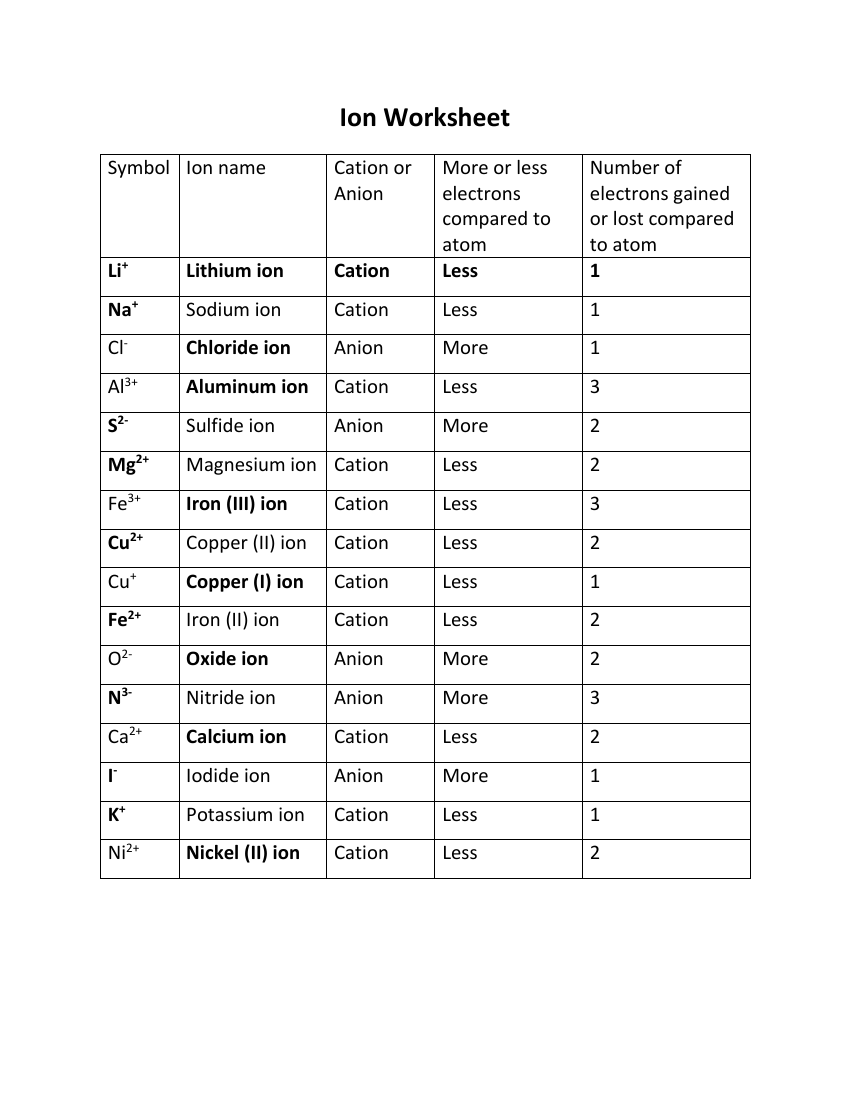
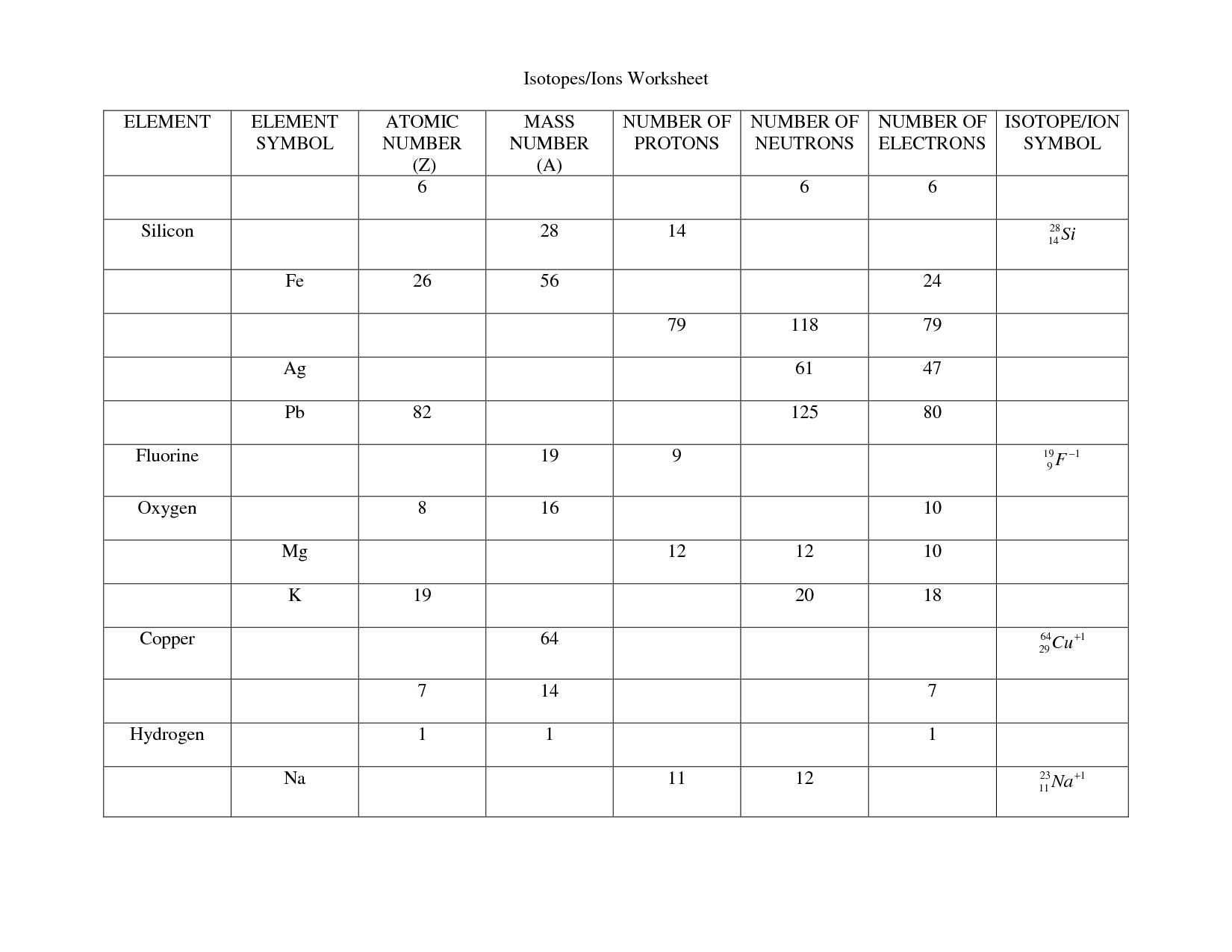
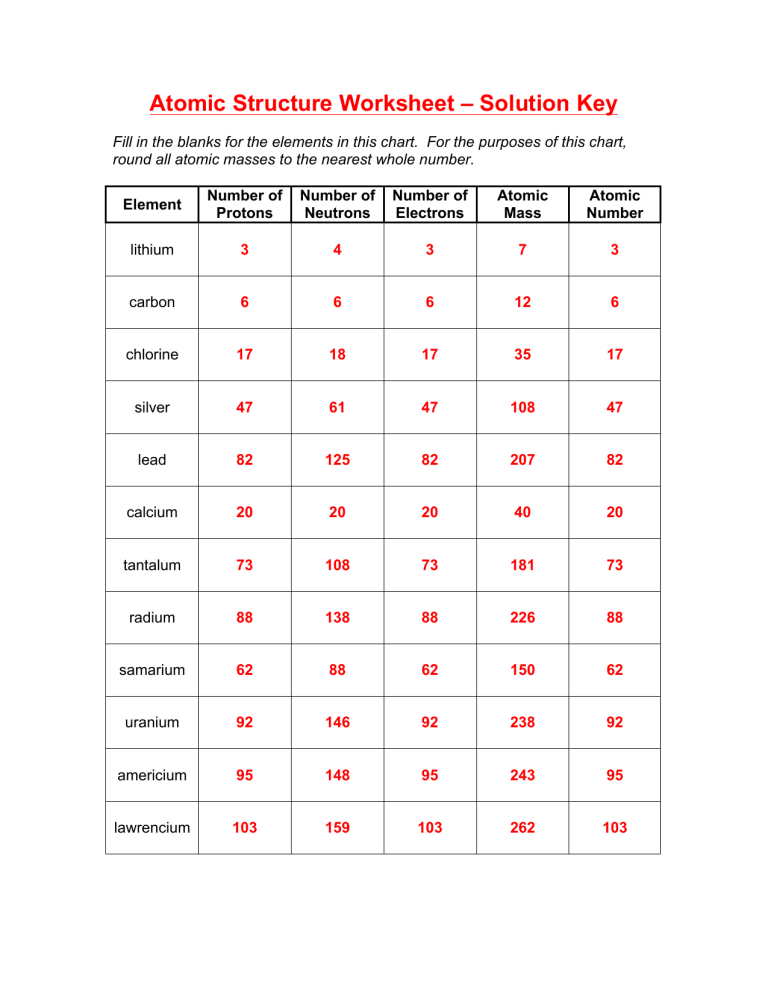
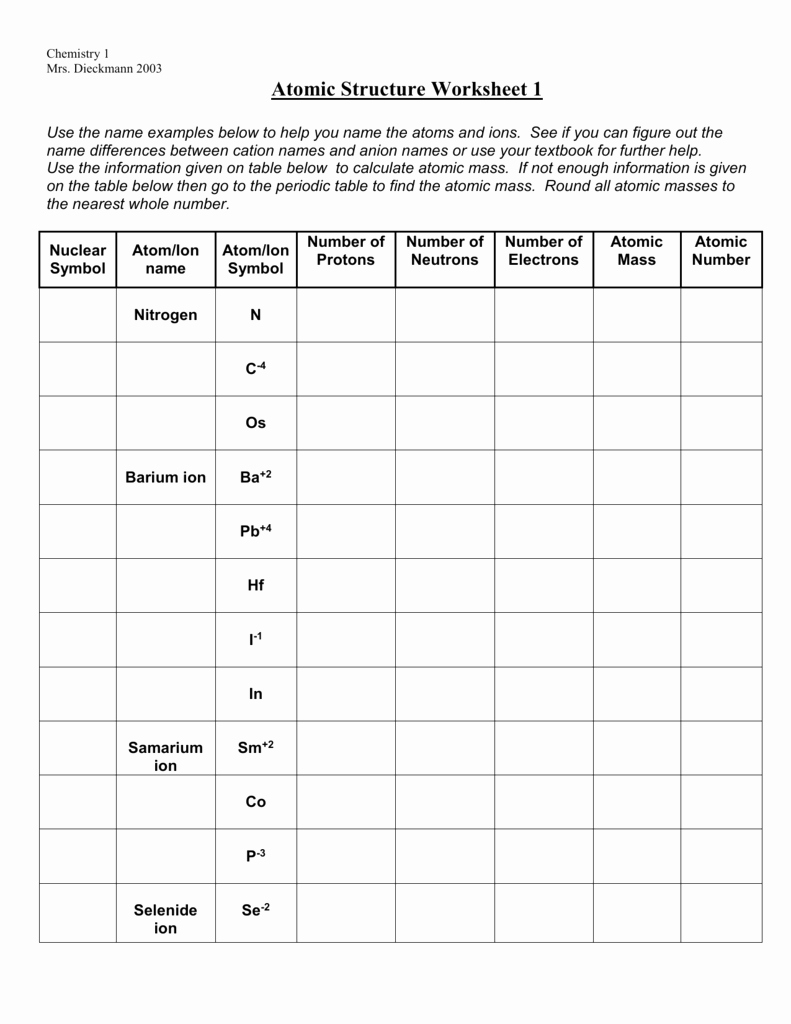
Atom Or Ion Worksheet Answers - A cation contains less electrons than a normal atom. To write the ion symbol, you must write the element symbol with the charge written on the top right. How do atoms become isotopes? For example, a sodium atom loses 1 electron to form a sodium ion na+. Answer the following questions below using your notes or your book.
For example, a sodium atom loses 1 electron to form a sodium ion na+. To write the ion symbol, you must write the element symbol with the charge written on the top right. A cation contains less electrons than a normal atom. Answer the following questions below using your notes or your book. How do atoms become isotopes?
How do atoms become isotopes? To write the ion symbol, you must write the element symbol with the charge written on the top right. For example, a sodium atom loses 1 electron to form a sodium ion na+. A cation contains less electrons than a normal atom. Answer the following questions below using your notes or your book.
11 Atom Worksheets With Answer Keys /
Answer the following questions below using your notes or your book. For example, a sodium atom loses 1 electron to form a sodium ion na+. How do atoms become isotopes? To write the ion symbol, you must write the element symbol with the charge written on the top right. A cation contains less electrons than a normal atom.
11 Best Images of Atom Worksheets With Answer Keys Atoms Ions and
For example, a sodium atom loses 1 electron to form a sodium ion na+. To write the ion symbol, you must write the element symbol with the charge written on the top right. How do atoms become isotopes? Answer the following questions below using your notes or your book. A cation contains less electrons than a normal atom.
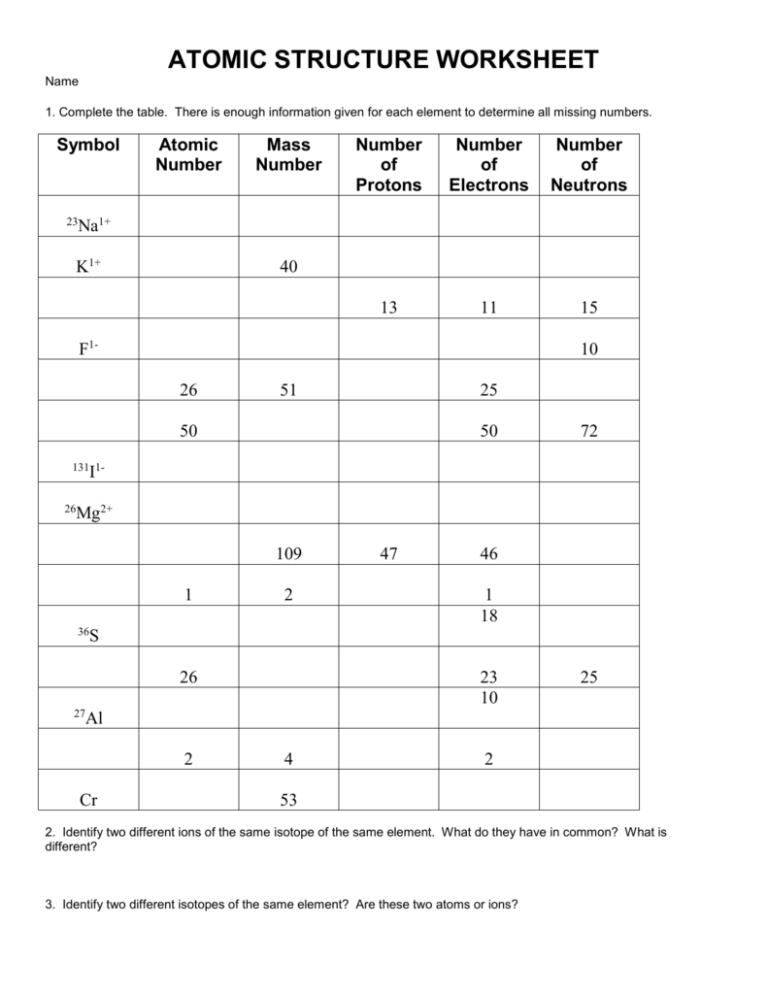
ATOMIC STRUCTURE WORKSHEET
For example, a sodium atom loses 1 electron to form a sodium ion na+. Answer the following questions below using your notes or your book. To write the ion symbol, you must write the element symbol with the charge written on the top right. A cation contains less electrons than a normal atom. How do atoms become isotopes?
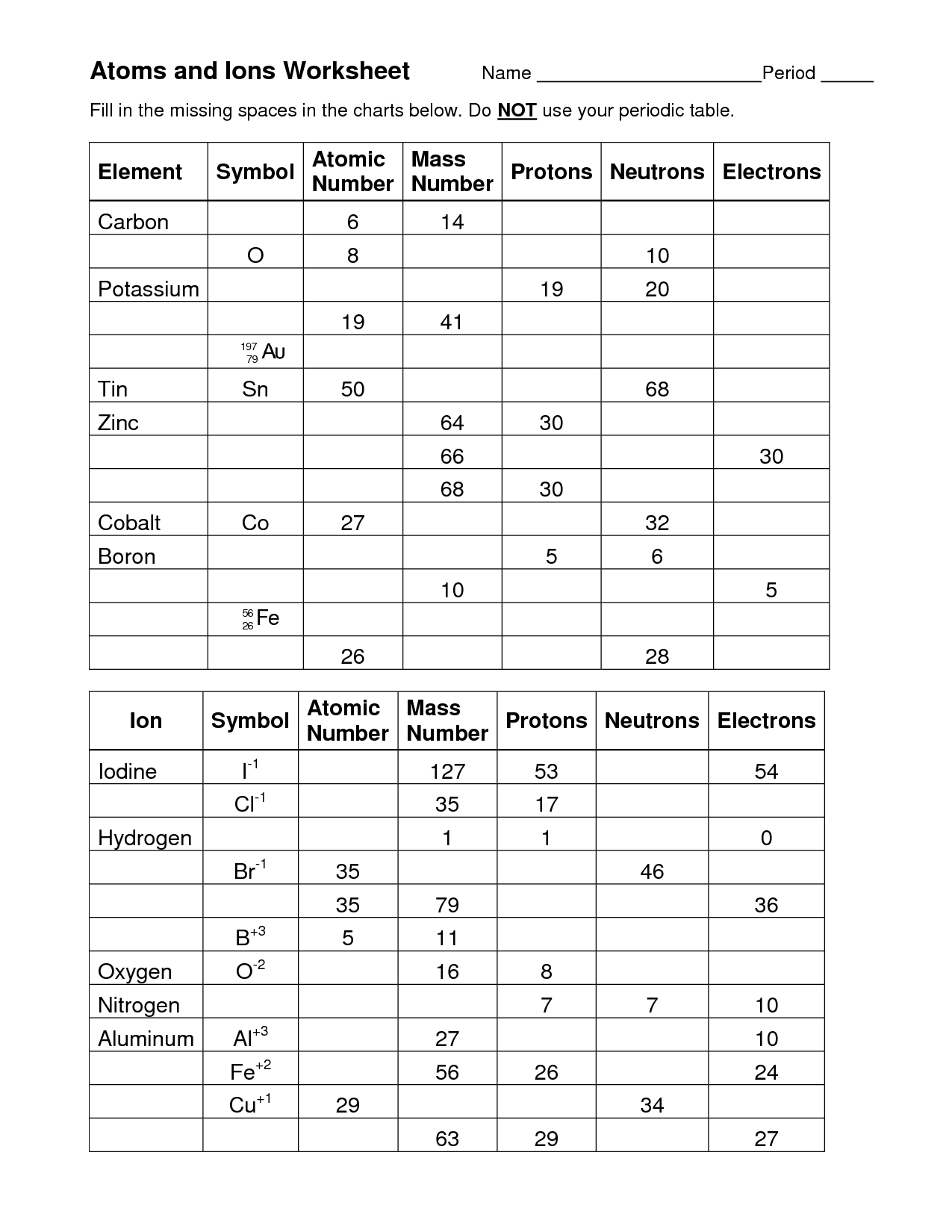
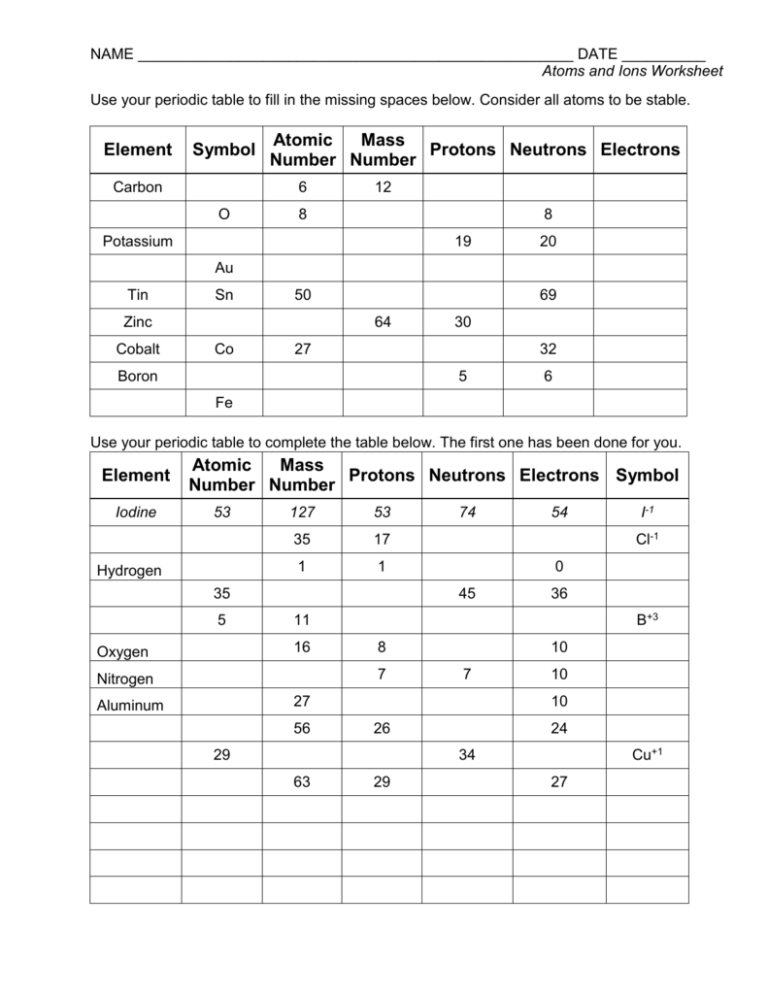
Atoms and Ions Worksheet
Answer the following questions below using your notes or your book. To write the ion symbol, you must write the element symbol with the charge written on the top right. For example, a sodium atom loses 1 electron to form a sodium ion na+. How do atoms become isotopes? A cation contains less electrons than a normal atom.
Ion Worksheet with Answers Science 7th Grade
How do atoms become isotopes? For example, a sodium atom loses 1 electron to form a sodium ion na+. Answer the following questions below using your notes or your book. A cation contains less electrons than a normal atom. To write the ion symbol, you must write the element symbol with the charge written on the top right.
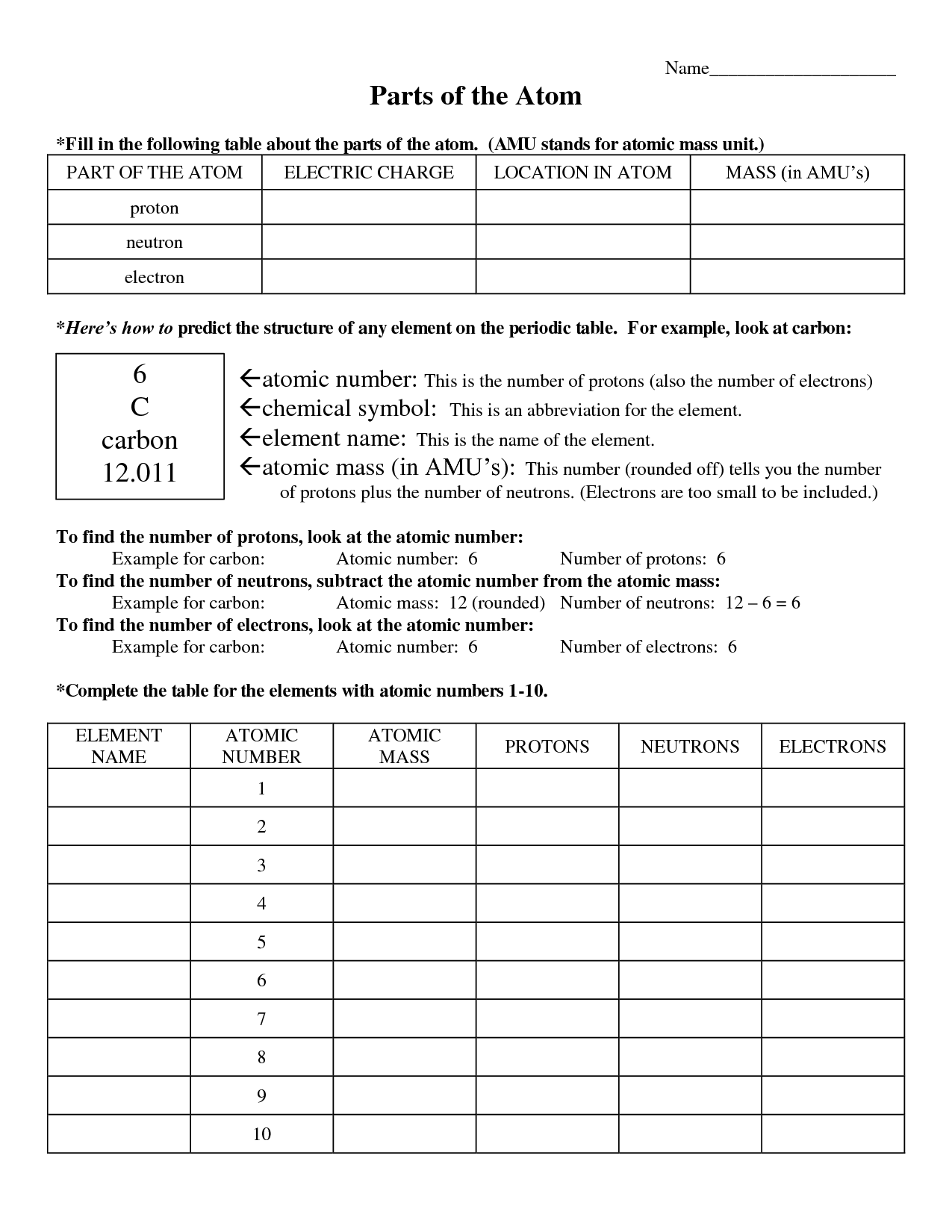
Basic Atomic Structure Worksheet 10++ Basic Atomic Structure
To write the ion symbol, you must write the element symbol with the charge written on the top right. Answer the following questions below using your notes or your book. How do atoms become isotopes? A cation contains less electrons than a normal atom. For example, a sodium atom loses 1 electron to form a sodium ion na+.
Atomic Structure Ions And Isotopes Worksheet
For example, a sodium atom loses 1 electron to form a sodium ion na+. Answer the following questions below using your notes or your book. A cation contains less electrons than a normal atom. To write the ion symbol, you must write the element symbol with the charge written on the top right. How do atoms become isotopes?
(PDF) Chapter 2 Worksheet / Atoms, Molecules, and Ions Contents and
To write the ion symbol, you must write the element symbol with the charge written on the top right. For example, a sodium atom loses 1 electron to form a sodium ion na+. Answer the following questions below using your notes or your book. A cation contains less electrons than a normal atom. How do atoms become isotopes?
Atomic Structure Worksheets Answers Chemistry
A cation contains less electrons than a normal atom. To write the ion symbol, you must write the element symbol with the charge written on the top right. For example, a sodium atom loses 1 electron to form a sodium ion na+. How do atoms become isotopes? Answer the following questions below using your notes or your book.
Atoms Vs Ions Worksheet Answer Key
How do atoms become isotopes? Answer the following questions below using your notes or your book. To write the ion symbol, you must write the element symbol with the charge written on the top right. For example, a sodium atom loses 1 electron to form a sodium ion na+. A cation contains less electrons than a normal atom.
A Cation Contains Less Electrons Than A Normal Atom.
For example, a sodium atom loses 1 electron to form a sodium ion na+. How do atoms become isotopes? To write the ion symbol, you must write the element symbol with the charge written on the top right. Answer the following questions below using your notes or your book.